Autoimmune Cytopenias

Section Editor
David Hughes, PharmD, BCOP
Boston Medical Center and Boston University
Case Study
COVID-19 Induced, Steroid-Refractory Immune Thrombocytopenia Treated With Novel Therapeutic Combinations
Presentation
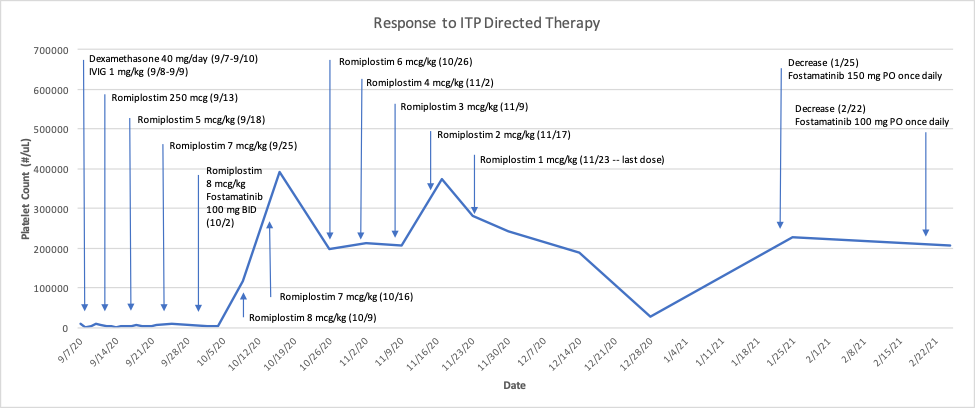
A 62-year-old male with a past medical history significant for hypertension, diabetes mellitus, hyperlipidemia, and a recent COVID-19 infection on 03/27/2020 presented to the emergency department on 9/7/2020 with hematuria and a platelet count of 9,000/µL (and a previously normal platelet count of 152,000/µL on 8/22/18). Hematology was consulted and he was initiated on dexamethasone 40 mg by mouth for 4 days, two doses of 1 g/kg intravenous immunoglobulin (IVIG), and 2 units of platelets with resolution of hematuria but persistent thrombocytopenia (see figure). Viral testing (hepatitis C and human immunodeficiency virus [HIV]) was negative and he had a positive antinuclear antibody (ANA) (1:80 titer).
Diagnosis and Treatment
A diagnosis of immune thrombocytopenia (ITP) was made and he was started on romiplostim 250 µg on 9/13/20 while admitted, in addition to corticosteroids. He was discharged on 9/22/20 on prednisone at 80 mg daily and for close follow-up and management by Hematology. He received increased doses of romiplostim at 5, 7, and 8 µg/kg over the next several weeks with no increase in platelet count (2,000/µL on 10/2/20). He was then started on fostamatinib 100 mg by mouth twice daily on 10/2/20 (in addition to current romiplostim dose). One week later, his platelet count increased to 117,000/µL.
Over the next several weeks, the romiplostim dose was gradually decreased weekly while he continued fostamatinib until cessation of romiplostim on 11/23/20. Concomitantly, he went through a gradual steroid taper until prednisone was discontinued. With normalized platelets, fostamatinib was decreased to 150 mg once daily on 1/25/21 (platelet count = 228,000/µL). One month later, his platelet count remained stable (207,000/µL) and fostamatinib was decreased to 100 mg once daily. To date, he has not had any episodes of bleeding and remains on fostamatinib as single-agent therapy with a plan for continued tapering as tolerated.
Discussion
This case illustrates several unique aspects of ITP management. First, ITP diagnoses have been documented previously after severe COVID-19 infections.1 However, optimal management in these cases is not well defined and these patients often become steroid-refractory as in the case reported here. Fostamatinib provided quick relief as an adjunct therapy to romiplostim and may have novel clinical applications given its potential utility in COVID-19.2,3 However, this remains unknown.
Second, the patient demonstrated steroid-, IVIG-, and thrombopoietin (TPO)-refractory disease. The median time of onset of response to romiplostim is usually 2 weeks after initiation4 and this patient’s platelet count did not respond within or even beyond that time window, warranting alternative therapy.
This patient demonstrated platelet response after transition to fostamatinib. Platelets remained stable even after incrementally decreasing the dose of romiplostim and steroid taper. There is a paucity of data regarding use of therapies with differing mechanisms of action in combination for both short- and long-term scenarios, although some experts would consider this approach in highly refractory cases.5 As patients become more resistant to currently available therapies, clinicians are seeking novel approaches to minimize bleeding and emergency department visits. Large, prospective trials are needed to confirm any hypotheses regarding the treatment approach discussed here but this case does demonstrate a successful transition/combination approach and treatment of a post COVID-19 infection diagnosis of ITP.
References
- Bomhof G, Mutsaers PGNJ, Leebeek FWG, et al. COVID-19-associated immune thrombocytopena. Br J Haematol 2020;190(2):e61-e64.
- Strich JR, Ramos-Benitez MJ, Randazzo D, et al. Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: A potential therapeutic. J Infect Dis 2020 Dec 24:jiaa789.
- Fostamatinib for hospitalized adults with COVID-19. Clinicaltrials.gov identifier NCT04579393.
- Hubulashvili D, Marzella N. Romiplostim (Nplate), a treatment option for immune (idiopathic) thrombocytopenic purpura. PT 2009;34(9):482-485.
- Miltiadous O, Hou M, Bussel JB. Identifying and treating refractory ITP: Difficulty in diagnosis and role of combination treatment. Blood. 2020;135(7):472-490.